CAR-T (chimeric antigen receptor ,CAR)therapy has become an important direction of cancer treatment. Commercial CAR-T is expensive, currently exceeds one million RMB (in China), and its therapeutic efficacy is not always satisfactory. CD (Suzhou) BioPharma Co., Ltd. has developed a new armored CAR-T - "PD-1Ab21-CD19CAR-T”. A clinical trial initiated by researchers at the Department of Hematology of a well-known hospital in China has been initiated. The preliminary results are very promising. At present, 3 patients with relapsed / refractory advanced B-cell lymphoma were treated with PD-1Ab21 CD19CAR-T infusion and all reached complete remission (CR), (see figure 1).
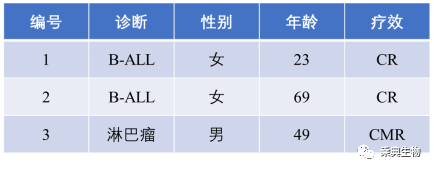
Fig. 1 Overview of the three patients enrolled in the group
Cases summary
Case 1 Female 23 years old, diagnosed in June 2021 with acute B lymphocytic leukemia, with TP53 mutation. Chemotherapy was received in the same year, but clinical outcome was not promising. In January 2022, the patient received autologous CD19CAR-T treatment. Although remission was achieved, relapse occurred after just a little over a month. In mid-March, an allogeneic CD19CAR-T cell therapy was received again And an allogeneic hematopoietic stem cell transplantation was followed after remission. But unfortunately, after only 4.5 months of transplantation, relapse happened again. In September 2022, the patient joined our PD-1Ab21-CD19CAR-T trial. Complete remission was achieved half a month later (CR), continued until the date of publication.
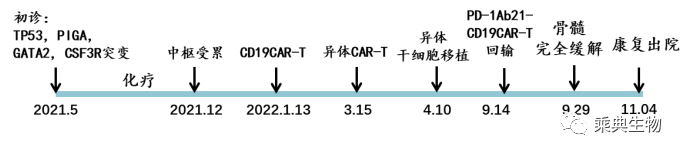
Fig. 2 diagnosis and treatment of case #1
Case 2 Female 69 years old, diagnosed as acute mixed cells (B / myeloid leukemia with central symptoms in mid-August, 2021, treated with chemotherapy for more than a year. In September 2022, he was re-admitted to hospital due to relapse and companion symptoms such as weakness of both lower limbs and brain distension. MRI examination of the brain showed that there were leukemic meninges, skulls and brain infiltration in the right temporal, left temporal, parietal and occipital regions. The patient was treated with PD-1Ab21-CD19CAR-T in late September 2022. And within half a month, no residual leukemic cells were detected in cerebrospinal fluid and bone marrow. And enhanced MRI showed that the brain tumor shrank significantly (to 1/6 size) one month later. Three months after infusion of CAR-T, MRI showed that the brain lesion completely disappeared and so that the patient got complete remission (CR). Due to the existence of blood-brain barrier, traditional CAR-T cells are usually not effective in the treatment of brain metastases. This patient showcased the great potential of PD-1Ab21-CD19CAR-T in patient with CNS metastasis.
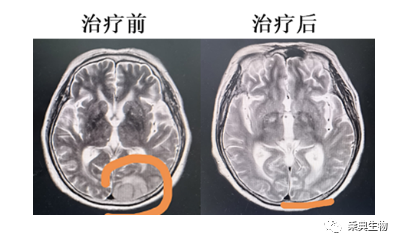
Fig. 3 Comparison of MRI results of case #2 with brain lesion before and after treatment
Case 3 Male 49 years old, diagnosed with diffuse large B-cell lymphoma (non-Hodgkin's lymphoma), Stage IVa (Ann Arbor) , aaIPI score 3, high risk PR. After patient received one cycle of chemotherapy, disease progressed and showed symptoms of brain invasion and mobility difficulties. Patient later also was treated with multi-cycle chemotherapy. In Nov. 2022, Patient received PD-1Ab21-CD19CAR-T cells and reached PR within 21 days and CMR in three months.
About PD-1Ab21-CD19CAR-T cells
Compared with the traditional CD19CAR-T cells, the PD-1Ab21-CD19CAR-T cells not only exert the function of normal CAR-T of killing tumor cells, but also secrete anti-PD-1/IL21 fusion protein(PD-1Ab21). This fusion protein can block the PD-1 inhibitory signal on CAR-T, also targeting IL21 to CAR-T cells, promote the proliferation of CAR-T cells, and significantly enhance its anti-tumor effect. At the same time, the fusion protein can also activate the patient's own anti-tumor T cell response (Bystander effect), which could prevent tumor recurrence. These design and results of PD-1Ab21-CD19CAR-T cells were well reflected and confirmed in the above three patient cases.

